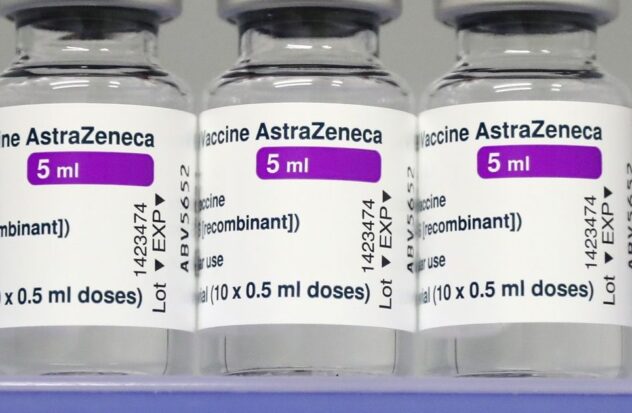
LONDON.-Pharmaceutical giant AstraZeneca has requested the withdrawal of European authorization for its COVID-19 vaccine, according to the European Union’s medicines regulator.
In an update published on Wednesday on the website of the European Medicines Agency (EMA), the regulator indicated that the approval of the Vaxzevria vaccine of the pharmaceutical company had been withdrawn “at the request of the marketing authorization holder.”
AstraZeneca’s vaccine against COVID-19 received approval from the EMA in January 2021. However, within a few weeks doubts arose about the safety of the drug, after dozens of countries suspended its use after detecting unusual blood clots in a small number of immunized people. The agency concluded that the AstraZeneca vaccine did not raise the overall chance of clots, but questions remained.
Partial results from its first large trial — which Britain used to authorize the vaccine — were marred by a manufacturing error that researchers did not immediately recognize. The lack of data on the vaccine’s effectiveness among older people led some countries to initially restrict its application to younger people, before lifting the limitation.
Millions of doses of this vaccine were distributed to poorer countries through a program coordinated by the United Nations, since its production and distribution was cheaper and easier. But later studies showed that the more expensive messenger RNA-containing vaccines made by Pfizer-BioNTech and Moderna provided better protection against COVID-19 and its many variants, and many nations switched to these options.
Source: With information from AP