An experimental drug against dengue, developed by Johnson & Johnson, showed good results in initial animal tests. JNJ-1802, as the researchers call it, proved to be effective against all four types of the virus in mice and prevented infection in monkeys of two types of the disease.
The positive results were announced this Wednesday (15/3), in an article published in Nature magazine.
This could be the first antiviral treatment for the mosquito-borne disease aedes aegypti. An estimated 390 million infections occur each year, resulting in 96 million symptomatic cases and 10,000 deaths.
Johnson & Johnson intends to develop the antiviral pill both for the treatment of dengue and to prevent infections.
Animal tests have shown that the drug blocks the action of two viral proteins, preventing the pathogen from making copies of itself.
Dengue is an infectious disease transmitted by the bite of the Aedes aegypti mosquito. With a higher incidence in the summer, its main symptoms are body aches and high fever. Considered a serious public health problem in Brazil, the disease can lead the patient to deathJoao Paulo Burini/Getty Images
 ***Photo-dengue-mosquito.jpg
***Photo-dengue-mosquito.jpgAedes aegypti has diurnal habits, can be found in urban areas and needs standing water to allow the larvae to develop and become adults, after the eggs hatch, within 10 daysJoao Paulo Burini/ Getty Images
 ***Photo-dengue-mosquito-2.jpg
***Photo-dengue-mosquito-2.jpgInfection of humans happens only with the bite of the female mosquito. Aedes aegypti transmits the virus through saliva when feeding on blood, which is necessary for eggs to be produced.Joao Paulo Burini/ Getty Images
 ***Photo-dengue-mosquito-3.jpg
***Photo-dengue-mosquito-3.jpgOverall, dengue has four serotypes. This means that a single person can be infected by each of these micro-organisms and generate permanent immunity to each of them. That is, it is possible to be infected up to four timesBloomberg Creative Photos/ Getty Images
 ***Photo-person-looking-thermometer.jpg
***Photo-person-looking-thermometer.jpgThe first signs are usually not specific. They appear about three days after the mosquito bite and can include: high fever, which usually lasts 2 to 7 days, headache, body and joint aches, weakness, pain behind the eyes, skin rashes, nausea and vomitingGuido Mieth/ Getty Images
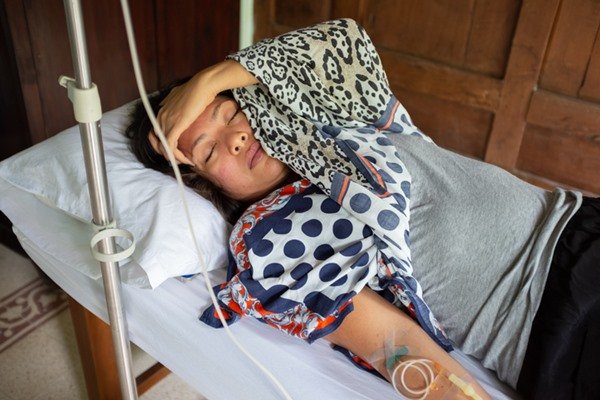 ***Photo-person-lying-on-hospital-gurney.jpg
***Photo-person-lying-on-hospital-gurney.jpgIn the period of decrease or disappearance of fever, most cases progress to recovery and cure of the disease. However, some patients may experience more severe symptoms, which include bleeding and can lead to death.Peter Bannan/Getty Images
 ***Photo-person-in-front-of-toilet-vomiting.jpg
***Photo-person-in-front-of-toilet-vomiting.jpgIn severe cases, the symptoms are: persistent vomiting, intense and continuous abdominal pain, or pain when the abdomen is touched, loss of sensitivity and movement, bloody urine, mucous bleeding, dizziness and pressure drop, enlargement of the liver and red blood cells or red blood cellsPiotr Marcinski / EyeEm / Getty Images
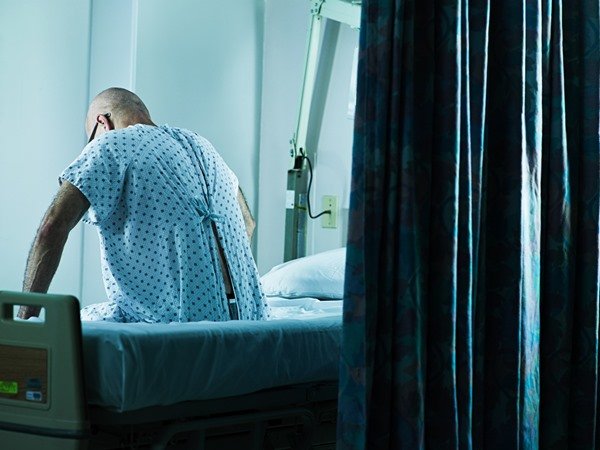 ***Photo-person-sitting-in-hospital-bed.jpg
***Photo-person-sitting-in-hospital-bed.jpgIn these cases, the symptoms result in shock, which occurs when a critical volume of blood plasma is lost. Signs of this state are clammy skin, rapid and weak pulse, agitation and low pressure.Image Source/ Getty Images
 ***Photo-person-lying-on-the-floor.jpg
***Photo-person-lying-on-the-floor.jpgSome patients may also have neurological manifestations, such as seizures and irritability. Shock is short-lived and can lead to death within 12 to 24 hours, or rapid recovery after appropriate antishock therapy.Getty Images
 ***Photo-person-holding-medicine-in-hands.jpg
***Photo-person-holding-medicine-in-hands.jpgDespite the seriousness, dengue can be treated with analgesics and antipyretics, under medical guidance, such as paracetamol or dipyrone to relieve symptoms.Guido Mieth/ Getty Images
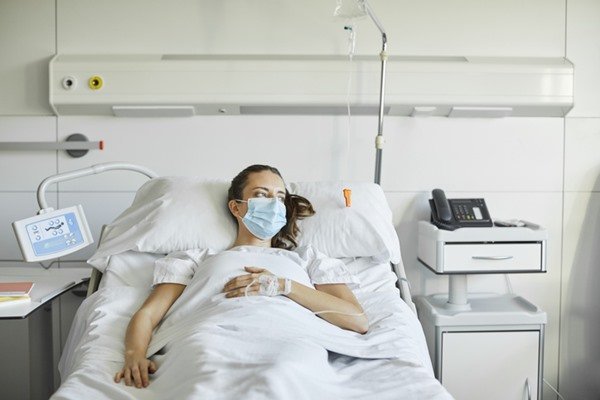 ***Photo-person-lying-on-hospital-stretcher-2.jpg
***Photo-person-lying-on-hospital-stretcher-2.jpgTo complete the treatment, rest and fluid intake is recommended. In the case of dengue hemorrhagic fever, therapy should be carried out in the hospital, with the use of medication and, if necessary, platelet transfusion.Getty Images
0
Clinical trials on humans have already started. The company reported, through a statement, that the treatment proved to be safe and well tolerated among human volunteers in the first phase and, now, a randomized clinical trial is being carried out.
“JNJ-1802 successfully completed a first phase 1 clinical trial in humans, with healthy volunteers, and was found to be safe and well tolerated. These findings support the clinical development of JNJ-1802, a first-class antiviral agent against dengue, which is now progressing into clinical trials for the prevention and treatment of dengue,” the researchers state in the paper.
Get news from metropolises on your Telegram and stay on top of everything! Just access the channel: https://t.me/metropolesurgente.
